Squamous Cell Carcinoma
Diphenylcyclopropenone (DPCP) also known as Diphencyprone has been employed for the treatment of cutaneous Squamous Cell Carcinoma (cSCC) lesions. Hapten Pharmaceuticals Samcyprone™ is a GMP produced proprietary DPCP gel that is available in 0.4% and 0.04% concentrations.
- Neo Adjuvant – Samcyprone™ can be employed to reduce a superficial spreading squamous cell carcinoma (cSCC) lesion. After successful reduction in field size the lesion can be surgically removed with reduced morbidity. Topical DPCP treatment is superior cosmetically to topical 5-fluorouracil (5-FU) treatment and also avoids the possible systemic side effects of 5-FU.
- Adjunct Therapy – Standard of Care Therapy for Stage III and Stage IV Squamous Cell Carcinoma often involves checkpoint inhibitors; specifically PD1 inhibitors. Samcyprone™ increases Tumour Infiltrating Lymphocytes (TIL’s) in the lesion microenvironment. Numerous clinical studies have shown that positive outcomes with PD1 therapy correlate with TIL levels at the tumour site.
- Samcyprone™ upregulates antigen presenting cells at the cSCC lesion site and involves the regional lymph nodes associated with the site. This activation of the acquired immune system can lead to direct antibody activity against HPV related cSCC’s.
- Adjuvant Therapy – Samcyprone™ can be employed at the surgically removed lesion site to continue TIL activity in the lesion microenvironment. This activity can reduce recurrence.
Regression of in-transit metastases of cutaneous squamous cell carcinoma with combination pembrolizumab and topical diphencyprone
Dina Poplausky, Jade N. Young, Brandon R. Block,Yeriel Estrada, Giselle K. Singer, Vicky Wong, Patricia Cabral,Yamato Suemitsu, Randie H. Kim, Philip Friedlander and Nicholas Gulati. Front. Oncol. 14:1294331.doi: 10.3389/fonc.2024.1294331
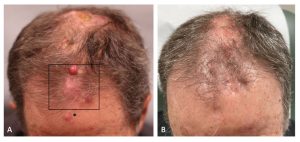
Figure 1 (A) The patient’s frontal scalp demonstrated erythematous nodules, consistent with in-transit metastases of cutaneous squamous cell carcinoma, before combination treatment with pembrolizumab and diphencyprone (DPCP). DPCP was applied to the boxed area. The asterisk denotes the area of sensitization. (B) Regression of the in-transit metastases after a 12-week course of combination treatment with pembrolizumab and DPCP.
We present the case of a 75-year-old male with cSCC and in-transit metastases on his scalptreated with the immune checkpoint inhibitor (ICI) pembrolizumab in conjunction with diphencyprone (DPCP), a topical hapten that induces a delayed-type
hypersensitivity reaction in the skin. The patient was enrolled in a clinical trial(NCT05481658) that involved the twice-weekly application of DPCP 0.04%ointment to four of the in-transit metastases on his frontal scalp, concurrent with pembrolizumab 300 mg administered every three weeks. Following effective sensitization and a twelve-week treatment course, complete clearance of all
lesions, DPCP-treated and non-DPCP treated, was achieved, with no adverse events. The immunologic profiles of the post-treatment biopsies were analyzed byTaqMan Low Density Array quantitative real-time polymerase chain reaction to measure immune marker gene expression. Relative to the non-DPCP-treated lesion, the DPCP-treated lesion demonstrated increased pro-inflammator genetic markers and T-cell activation. This case represents the first reported instance of in-transit metastases of cSCC successfully treated with DPCP and an ICI. It highlights the potential safety and efficacy of DPCP with systemic immunotherapy for the management of in-transit metastases of cSCC in patients for whom surgery and radiation may be contraindicated.

