Cutaneous Melanoma
- Neo Adjuvant Therapy – Samcyprone™ can be employed to reduce a superficial spreading melanoma lesion. After successful reduction in field size the lesion can be surgically removed with reduced morbidity
- Adjunct Therapy – Standard of Care Therapy for Stage III and Stage IV Melanoma often involves Checkpoint Inhibitors specifically PD1 inhibitors. Samcyprone™ increases Tumor Infiltrating Lymphocytes (TIL’s) in the lesion microenvironment. Numerous clinical studies have shown that positive outcomes with PD1 therapy correlate with TIL levels at the tumor site.
- In-Transit Metastases – Samcyprone™ (DPCP) has an established success rate of clearing satellite metastatic lesions in Stage III and Stage IV Melanoma.
- Adjuvant Therapy – Samcyprone™ can be employed at the surgically removed lesion site to continue TIL activity in the lesion microenvironment. This activity can reduce recurrence.
Molecular Profiling of Immune Activation Associated with Regression of Melanoma Metastases Induced by Diphencyprone
Nicholas Gulati, Sandra Garcet , Judilyn Fuentes-Duculan , Patricia Gilleaudeau , Mary Sullivan-Whalen , Xuan Li , Mayte Suárez-Fariñas , Daniel G Coit , James G Krueger. J Invest Dermatol. 2016 Oct;136(10):2101-2103.
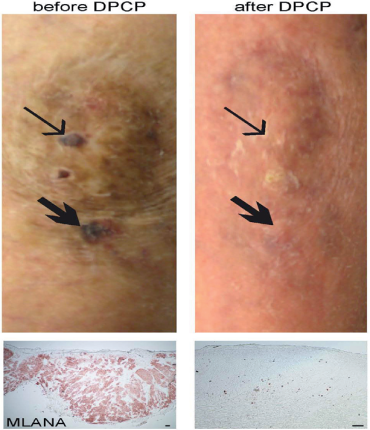
We present results from five of six patients treated with DPCP, excluding patient 001, who left the trial before a delayed-type hypersensitivity reaction could be successfully induced (see Supplementary Materials and Methods and Supplementary Table S1 online). All five patients showed partial or complete regression of their cutaneous metastases upon DPCP treatment (see Figure 1 for an example of complete clinical and MLANA immunohistochemical response). Our finding that DPCP applications significantly increased PD-1 expression is potentially of great clinical relevance, because inhibitors of PD-1 are currently the standard-of-care treatment for melanoma (Ribas et al., 2015). We have previously shown that DPCP leads to increases in CD8 T cells, as well as PD-1, PD-L1, and PD-L2 in healthy volunteers (Gulati et al., 2014). Therefore, there is the potential for synergy between DPCP and PD-1 checkpoint inhibition. Patient 004 was having minimal regression of his bulky cutaneous metastases while solely receiving either DPCP as part of our trial or the PD-1 inhibitor pembrolizumab, but showed dramatic regression when they were combined. This supports the hypothesis that these two therapies might complement each other, but a larger trial with more patients is needed.
Topical immunotherapy with diphencyprone for in transit and cutaneously metastatic melanoma
Diona L Damian 1, Robyn P M Saw, John F Thompson. J Surg Oncol. 2014 Mar;109(4):308-13
Topical diphencyprone (DPCP) can be used to treat in transit and cutaneously metastastatic melanoma. To date, 50 patients have received DPCP therapy for at least 1 month at our institution, with complete clearance of cutaneous disease in 46% and partial response in a further 38% of patients. Topical immunotherapy with DPCP is inexpensive and well-tolerated and should be considered in patients with skin metastases unsuitable for or refractory to other forms of therapy.
Definite regression of cutaneous melanoma metastases upon addition of topical contact sensitizer diphencyprone to immune checkpoint inhibitor treatment.
Nicholas Gulati, Richard D. Carvajal, Michael A. Postow, Jedd D. Wolchok and James G. Krueger
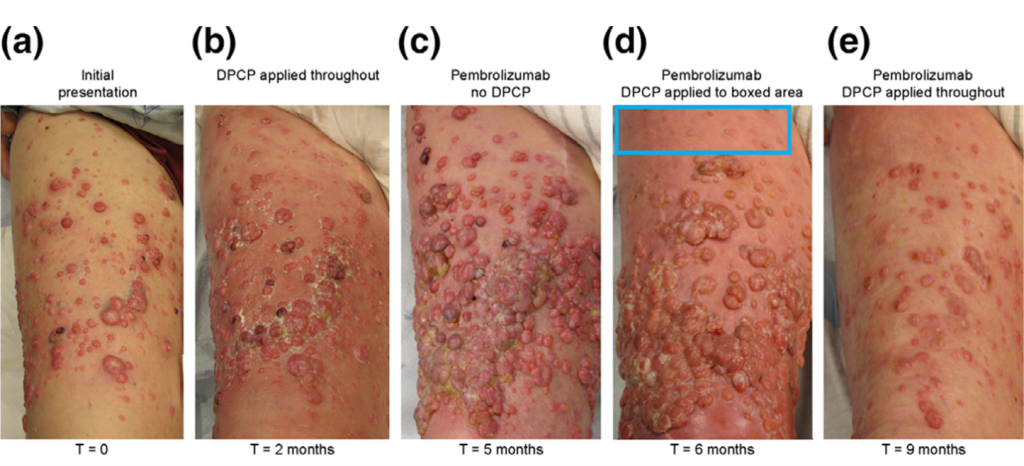
Figure 1. Responses of cutaneous melanoma metastases to topical DPCP, pembrolizumab and a combination of the two. A pretreatment photograph of the patient’s right anterior thigh (the advancing front of his metastatic disease) shows numerous amelanotic cutaneous melanoma metastases (Panel a). Two months of topical DPCP applications (Panel b) led to an incomplete tumor response, while three months of pembrolizumab therapy without DPCP (Panel c) resulted in rapid progression of disease.
Topical DPCP added to background pembrolizumab therapy, first to a select area for one month (Panel d), and then throughout the area for an additional three months (Panel e), led to dramatic regression of cutaneous melanoma metastases.

